Topic 2 zooms in even more than in the previous topic and investigates how molecules interact to sustain life. It focuses primarily on the molecular structures of the nutrients, and how they bond together to make bigger structures. A section about DNA structure, enzymes, cellular respiration, and photosynthesis is also included, which I will cover more in depth in topic 7 and 8. This topic may seem intimidating to some, but it is really not that bad since the structures follows rules that makes them easy to identify. The most challenging part is probably drawing the structures, with the different types of monosaccharides being the most difficult to remember. Fortunately, there are methods which I will cover later that makes remembering them easier. My tip for this chapter is just to visualize the structures, and to practice drawing them.
Points for revision:
- DNA replication, transcription, and translation
Drawing structures
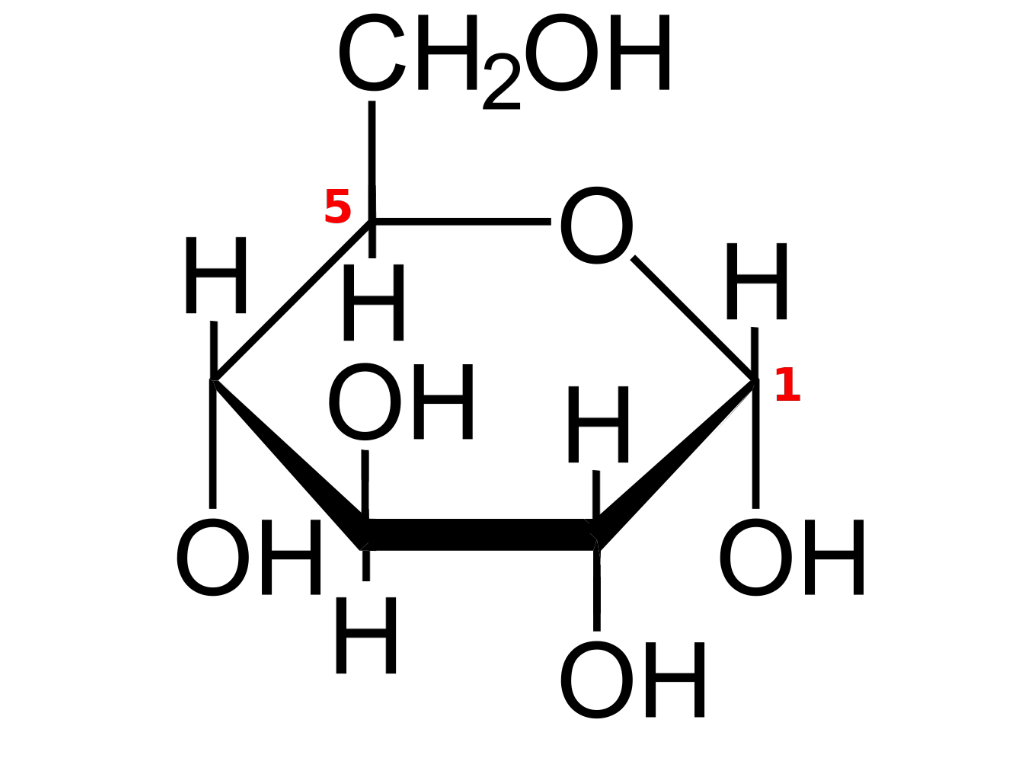
The recipe for drawing molecular structures for carbohydrates, is to start with the carbon skeleton. It is usually a ring of carbons with one oxygen. After the carbons are drawn, add the hydroxyl groups (OH) to the carbons. The placement of the OH groups can determine the identity of the carbohydrate. The final step is to add the remaining hydrogens wherever there is space. All carbons in a carbohydrate are usually bonded to a hydrogen. Since carbon has 4 valence electrons, it wants 4 more electrons to fill its outer shell. A line is used to represent a pair of electrons in a covalent bond, so any carbon atom can have 4 lines going out of it.
Identifying structures
It is quite simple to identify the structures of the nutrients. If it is circular, it is most likely a carbohydrate, if it is linear, it is most likely a fatty acid, and if it has an amino group (NH2), it is a protein. This holds as far as this chapter is concerned.
Carbohydrates
The smallest units of carbohydrates are called monosaccharides, with mono meaning “one”. The word carbohydrate can be split into two parts, Carbo, and hydrate. Carbo indicates that carbons are present, and hydrate indicates that water is present. Even though water is not directly present, it makes it easier to make sense of the general formula of a monosaccharide: CnH2nOn. If you look at the hydrogen and oxygen part, you can see that it does resemble the chemical formula of water.
Monosaccharides can bond together into a disaccharide in a process called a condensation reaction. In a condensation reaction two hydrogen atoms and one oxygen atom are released as water, bonding the first carbon and the fourth carbon together with a central oxygen in a covalent bond. The opposite reaction of a condensation reaction is called hydrolysis. In hydrolysis water is added, breaking up the disaccharide into two monosaccharides.
Polysaccharides are long chains of monosaccharides, and usually comes in the form of cellulose, starch, or glycogen. Cellulose is a long straight chain of glucose, and it is cellulose that makes plant cell walls rigid. Starch and glycogen are ways to store monosaccharides in plants and animals. Starch is only produced in plants, and glycogen only in animals.
The structures expected to be known are alpha glucose, beta glucose, and ribose. Alpha and beta glucose are 6 carbon isomers, and the only difference is that the hydrogen and hydroxyl group (OH) bonded to carbon number one is flipped. Ribose however is a 5-carbon structure, and it is one of the main molecules in DNA.
Lipids
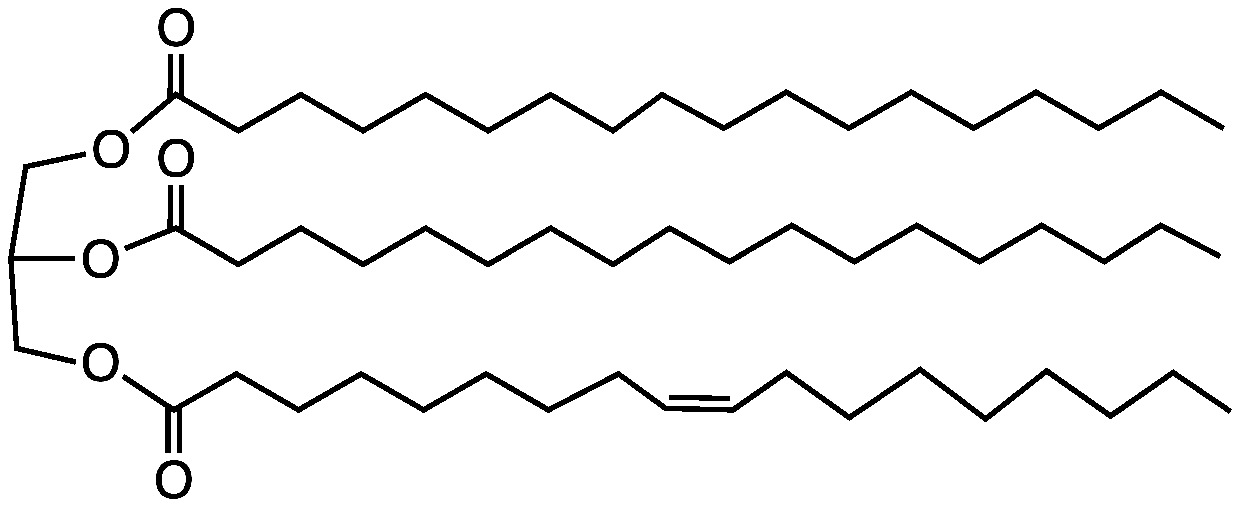
A triglyceride lipid is made of one glycerol and three fatty acids with the fatty acids bonded to the glycerol through a condensation reaction. The glycerol always stays the same, but the fatty acids can vary. A fatty acid is a long straight chain of carbons squeezed between a carboxyl group (COOH) and a methyl group (CH3). Fatty acids are divided into saturated and unsaturated, and its identity depends on the bonding between the carbons in the carbon chain.
A saturated fatty acid is completely straight because it does not have any double bonds. The name saturated comes from the fact that it is saturated of hydrogen since every carbon in the carbon chain has two hydrogens bonded to it. Saturated fats are solid at room temperature, and it not recommended to consume too much of it since it can be harmful in large quantities.
An unsaturated fatty acid, however, has at least one double bond in the carbon chain. A fatty acid with more than one double bond is called a polyunsaturated fatty acid. Unsaturated fats have a bend at the double bond, which prevents them from packing too tightly together, making them liquid at room temperature. Polyunsaturated fats can be divided into cis and trans fats. Cis fats are natural unsaturated fats and are considered the healthiest. Examples of cis fats are omega three and omega 6. Olive oil is high in cis fats. The other kind of polyunsaturated fat is trans-fat. You can think of it as transformed fats, since the double bonds have been through a process of hydrogenation, thus straightening them out. Trans fats should also be avoided as much as possible since they can have the same effect as saturated fats.
Proteins
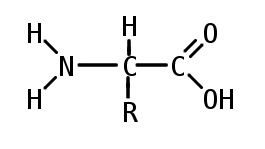
Proteins are made of smaller units called amino acids. There are 20 amino acids coded for by codons, which will be explained in further detail in topic 7. Among the 20 amino acids, 9 of them are essential, meaning that they have to be consumed since the body does not produce them. Amino acids follow a general formula, with a central carbon between an amino group (NH2) and a carboxyl group. The R group bonded to the central carbon is the varying group. It is the R group that determines what kind of amino acid it is.
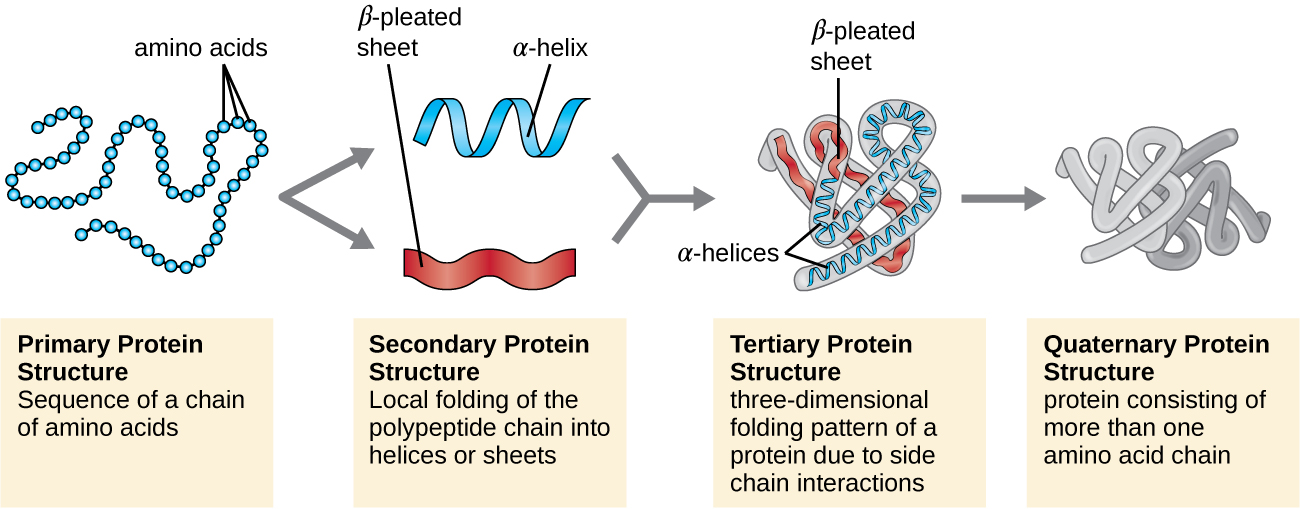
Proteins can be ordered into 4 levels of organization. The primary structure is a chain of amino acids covalently bonded by a condensation reaction called a polypeptide chain. The secondary structure is when the chain either forms a sheet or a helix held together by hydrogen bonds. It is the primary structure that determines how the next levels are formed. The tertiary structure is three dimensional, created by the attractions in the secondary structure. It is often depicted as a curled-up sausage. The highest order of organization is the quaternary structure. It is a big structure made up of multiple polypeptide chains which sometimes includes non-polypeptide groups. An example of a quaternary protein is hemoglobin.
Enzymes
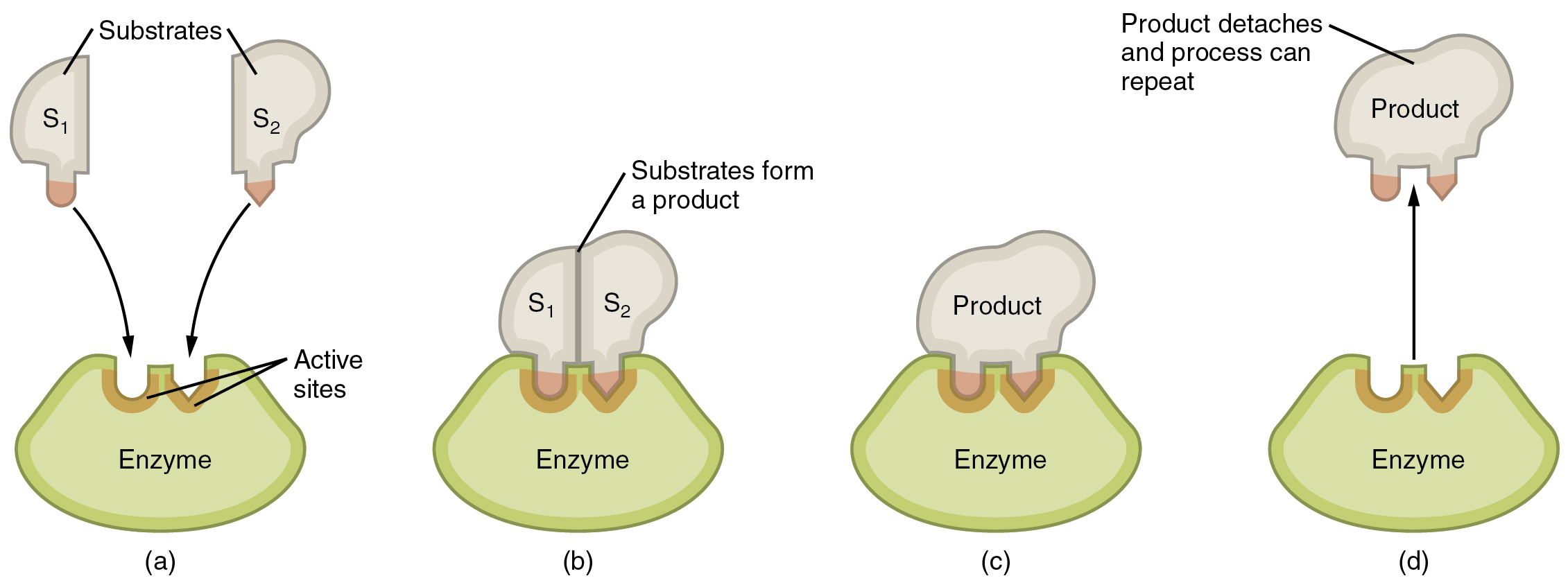
Enzymes are proteins that work by lowering the activation energy needed for a reaction. Enzymes are usually quite big and complex proteins with a three-dimensional shape and an area called the active site. It is the active site that binds with the substrate that will undergo a reaction. The active site and the substrate can be thought of as a lock and key. Both must correspond for the system to work. The substrate has to have the right shape and charge to be able to combine with the corresponding enzyme. Enzymes can be influenced by factors such as temperature, PH, and substrate concentration. When temperature increases, the enzyme reactivity will increase as a result of more kinetic energy. It will increase until it reaches a maximum, where the temperature is so high that the enzymes start to denature. Further increased temperature will then quickly reduce the reactivity. Enzymes also usually have an optimal PH, with a range below and above where the enzyme still can function. Any PH outside of that range will stop the enzyme from functioning. Finally, reactivity can also be controlled by substrate concentration. As substrate concentrations increase, the reactivity will increase as well, as there are more collisions between enzyme and substrate. However, at a given point, the reactivity will reach a plateau, where it flattens out since all of the enzymes are working at a max rate.
Properties of water
Water is made by one oxygen atom and two hydrogen atoms with covalent bonds between them. The difference in electron negativity in the O-H bond creates a dipole which makes water molecules polar. The polarity and structure of the molecule is important because it gives water many of its properties detrimental to life. One property that is very special to water is that it is less dense in its solid form than its liquid form, because the water molecules form crystals in a specific formation. Water being less dense in its solid form allows ice to float on top of water, which is important for marine life.
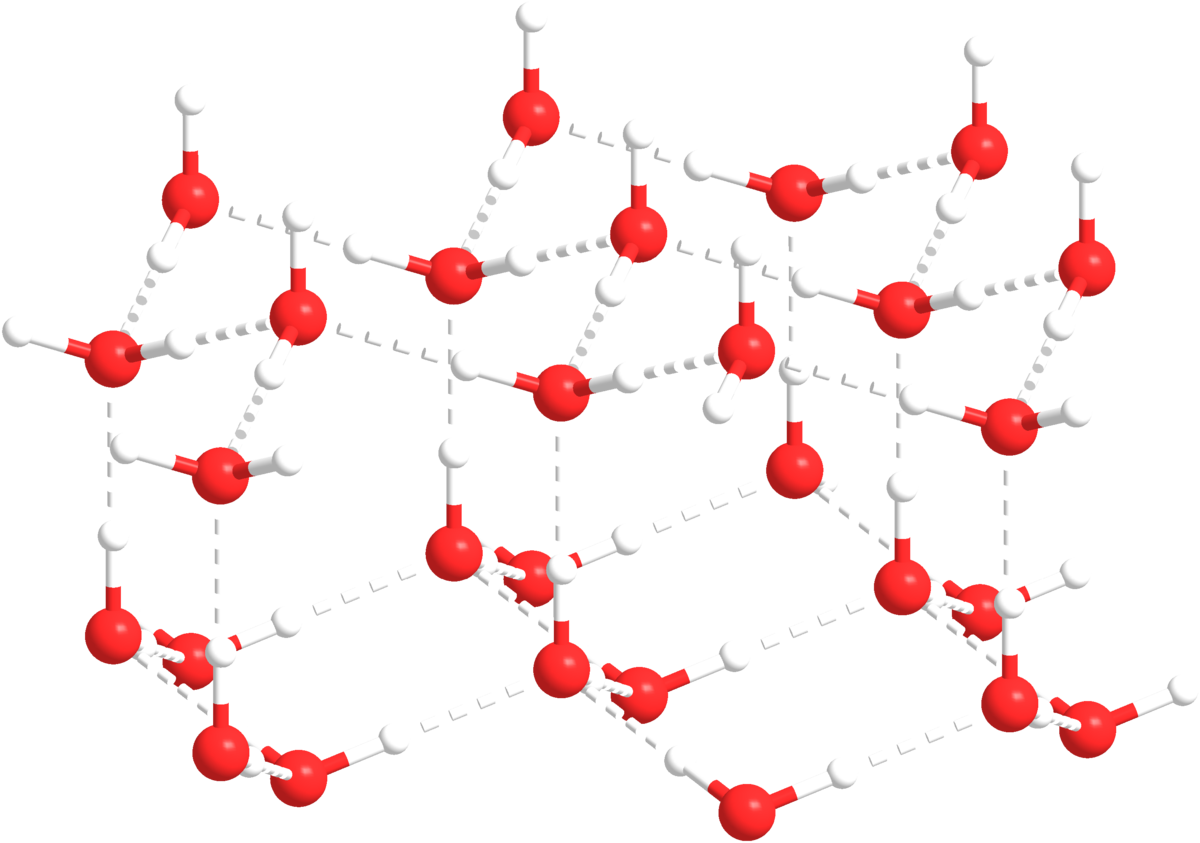
Other properties of water are its adhesive and cohesive properties. Polar molecules like to stick to other polar molecules, which means that there is an attraction between water and any other polar molecule, which leads to cohesion. Because water itself is also polar, the attraction between molecules (hydrogen bonds) causes water to stick to itself as well. Surface tension is a good way to demonstrate this. The polarity of water also means that water is an excellent solvent. You may have heard that like solves like. This means that polar molecules can dissolve polar molecules, and that un-polar molecules can dissolve un-polar molecules. Finally, water has a high specific heat capacity and latent heat capacity of evaporation, which means that it takes a lot of energy to heat up and vaporize. This means that water can keep its temperature relatively stable, and that we can transfer heat out of our bodies by heating up water and releasing it as vapor.
DNA and RNA structure
Deoxyribonucleic acid or DNA is a double helix made of a sugar phosphate backbone, and a nitrogenous base. The backbone is made of a 5-carbon compound called ribose, and a phosphate. Each ribose is also bonded to a nitrogenous base. There are 4 nitrogenous bases in DNA: Adenine(A), Thymine(T), Guanine(G), and Cytosine(C). A bonds with T, and G bonds with G. This is because A and T can have two hydrogen bonds, while C and G can have three. It is therefore much more energetically preferred for them to utilize their bonds. A ribose bonded to a phosphate and a nitrogenous base is called a nucleotide. DNA is just two long chain of nucleotides twisted around each other in a helix.
RNA is similar to DNA, but there are three distinct differences. Firstly, RNA is single stranded. This means that it only has one strand, and no helix structure. Secondly, RNA has Uracil(U) instead of Thymine. In RNA adenine therefore bonds with uracil instead of thymine. Lastly, the ribose in RNA has one oxygen more. That is the reason why DNA is called “Deoxy”ribonucleic acid. It is deoxidized.

DNA replication, transcription and translation
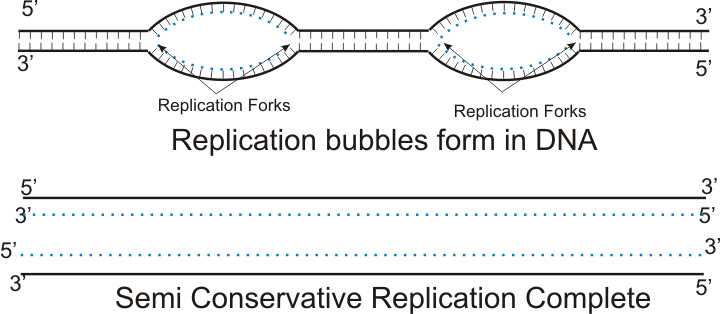
DNA replication happens in the S phase of interphase, where the genetic material duplicates. It starts with an enzyme called helicase unwinding the DNA into two single strands. Another enzyme called DNA polymerase then comes in behind and adds nucleotides to the two separated strands by complimentary base pairing, thus creating two new strands. These two new strands then both twists around the strand which it was copied from, forming two strands of identical DNA. Notice that a new strand is twisted around an old strand. This is called semi-conservative replication, meaning that some of the old DNA is always conserved.
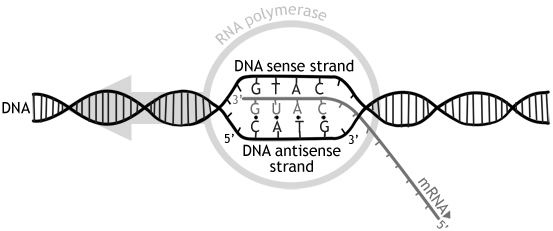
The whole point of DNA is to code for protein. However, the DNA is in the nucleus, while the ribosomes are in the cytoplasm. DNA therefore has to be transcribed over to RNA to be transferred out of the nucleus to the ribosomes. This process is called transcription. In transcription, an enzyme called RNA polymerase reads off one of the DNA strands, but instead of adding DNA nucleotides, it adds RNA nucleotides. This means that is uses uracil instead of thymine. The resulting strand of RNA is called a mRNA (messenger RNA). It is called mRNA because it is like the messenger between the nucleus and the ribosomes.
After the mRNA has reached a ribosome, a process called translation begins. Translation does as the name suggests, translate DNA into the language of amino acids. DNA is read by the ribosome in triplets of three and three bases (called codons), where each codon corresponds with an amino acid. The molecule that transfers the amino acids to the ribosome is called a tRNA (transfer RNA). It has the shape of a three clover, and at the top clover it has an anti-codon that corresponds to the DNA code and an amino acid. If the DNA codon reads AAC, the corresponding tRNA anti-codon will read UUG, and the amino acid corresponding to UUG is leucine. A ribosome has three translation units, and the translation process starts when the first codon enters the first unit. A corresponding tRNA brings the appropriate amino acid, and the ribosome moves to the next codon, moving the first codon and tRNA to the second unit. The ribosome then reads the next codon, and a tRNA brings the appropriate amino acid. When the ribosome moves again, the first amino acid is bonded to the second amino acid, which is bonded to the tRNA at the first position. When a third codon enters it pushed both the previous codons one unit, thus pushing the tRNA with the polypeptide chain to the second position. The polypeptide chain will never reach the third position, since the tRNA at the third position is now ejected from the ribosome unit. This process will continue until the whole mRNA chain is read. When the last codon is read, the polypeptide chain is released, and it becomes a protein.
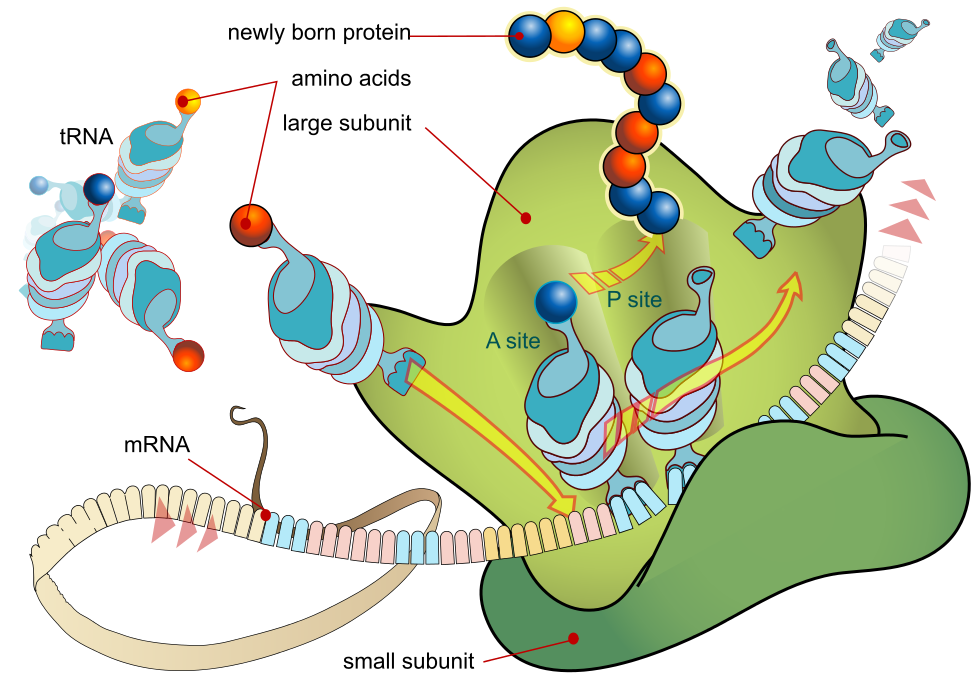
I would watch this animation to get a more visual understanding: https://www.youtube.com/watch?v=5bLEDd-PSTQ
A more in-depth explanation of these processes is covered in topic 7
Respiration
Respiration is the process that turns glucose into energy in the form of ATP. Respiration can be divided into aerobic and anaerobic respiration. Anaerobic respiration means that it can occur without the presence of oxygen. Both pathways start with a process called glycolysis, where a 6-carbon molecule (glucose) is split into two 3-carbon molecules called pyruvate. The pyruvate then enters either aerobic or anaerobic respiration. Anaerobic respiration happens differently in different organisms. In procaryotic cells such as yeast, the pyruvate is decarboxylated into the 2-carbon molecule ethanol. While in humans, the pyruvate is simply made into lactic acid (3 carbons), which can be stored until oxygen is present again, reversing the reaction so it can go down the aerobic path. The aerobic path is a little more complicated, but it is the most efficient path. Anaerobic respiration does not generate a lot of ATP, but aerobic respiration does. This is because the pyruvate goes through some reactions to become a compound called acetyl-CoA, which enters the Krebs cycle. The Krebs cycle itself does not produce a lot of ATP, but the biproducts produce a lot of ATP in the last step of aerobic respiration called the electron transport chain.
An in-depth explanation of respiration can be found in topic 8.
Photosynthesis
Photosynthesis is the process which turns inorganic carbon compounds into organic glucose. It occurs in the chloroplasts of plants cells when the chlorophyll in the chloroplasts absorbs energy from the sun. Photosynthesis happens in two stages, the light dependent and the light independent stage. In the light dependent stage, the energy from the sun goes through a series of reactions, and ends up with ATP and another molecule, which moves to the light independent reaction. In the light independent reaction, the molecule and ATP is used to turn carbon dioxide into glucose.
An in-depth explanation of photosynthesis can also be found in topic 8.
Helpful videos:
Metabolism and nutrition: https://www.youtube.com/watch?v=fR3NxCR9z2U
Enzymes: https://www.youtube.com/watch?v=ok9esggzN18
DNA structure: https://www.youtube.com/watch?v=o_-6JXLYS-k
Photosynthesis and respiration: https://www.youtube.com/watch?v=xmfhKbmQhq0
I experienced this chapter pretty reliant on memorization, but otherwise it was a fun chapter. I did not find it too difficult because I was familiar with the material beforehand. Many of the experiments introduced were very interesting. I especially liked the experiment done by Meselson and Stahl, proving the semi-conservative replication of DNA.